Table of Contents
Battery

The battery is the main part of the electrical system in an automobile. Without battery, engine cannot be started with the starting motor.
The battery supplies current to various part of the automobile vehicle such as for starting motor, ignition system, head and tail light, brake light, to wiper and other accessories.
A car battery is a rechargeable battery used to jump start a motor vehicle.
Its primary purpose is to provide an electrical current to the electric starter motor, which in turn ignites the chemically powered internal combustion engine that actually drives the vehicle.
Type
The type of batteries are
1) Lead-Acid battery
2) Alkaline battery
This is also two type
a) Nickel iron type
b) Nickel cadmium type
3) Zinc-air battery
Vehicle batteries should be robust construction since they are are to withstand sever vibration in addition to the high charging rate as well as heavy discharge convent required for different automobile service.
The construction with component of lead acid battery is explain below.
Function of Batteries
- Battery supply high current to starter motor and low current to ignition system.
- It supplies current to accessories which operate electrically.
- Without Battery Engine Wont Fire
- It stores the electrical energy and control the voltage regulation of electrical system.
- Works With The Alternator to Power Electronics
- The battery helps regulate voltage
Lead-Acid battery

Construction
The lead acid battery is widely used in automobile vehicle.
It consist of following component –
1) Container
It is constructed in single piece and made of acid resistance of hard rubber or plastic or bituminous composition.
It is divided by partitions into compartments for individual cell.
Ribs are there at the button of each compartment. The battery plates rest on these ribs.
The space between the ribs are provided to collect sediments. This minimizes the danger of short circuit due to sediment.
2) Grids and Plates
The battery plates are made of a lead alloy containing 6 to 8% antimony, which makes them resistant to electrochemical corrosion and gives them a strength and rigidity.
The plates are in the form of grids. Number of plates of positive and negative types and separators are there in a cell. Positive plates are kept one less than the negative plate in order to make use of all surface of the positive plate.
On the plate grids, lead oxide paste is applied. Then plates are assembled into a battery.
The cells are filled with electrolyte and given an initial charge.
This changes the lead oxide paste on the negative plate to sponge lead which is grey in colour and changes the lead oxide paste on the positive plate to lead peroxide which is brown in colour.
The plates are properly spaced and welded to a lead antimony plate strap. This form a plate group.
Each cell is composed of negative and positive plate group and separator.
The negative and positive plate are arrange alternatively.
3) Separator
Separators are placed between the negative and positive plates. This prevents the positive and negative plates from direct contact with each other,resulting internal short circuit.
Also they may be porous to permit electrolyte to circulate between the plates.
The separator are made of wood, spun glass, porous rubber sheet, glass fibre or resin impregnated fibre, PVC of porous plastic.
Some batteries have separators made of polyvinyl chloride or polyethylene saturated cellulose.
Separators have ribs on the side facing the positive plate provide greater electrolyte volume to next to the positive and improve efficiency by increasing electrolyte circulation.
4) Cell cover
Each cell is sealedby a cover of hard rubber through which the positive and negative terminals are projected.
Adjacent negative and negative terminal are connected by cell connector strap.
It’s cover have an opening for filling the electrolyte and a filler cap is provided on this opening with an air vent to scape the gases.
5) Electrolyte
The sponge lead and lead peroxide which fill the respective plate.
Electrolyte is a chemically pure sulphuric acid diluted with distilled water.
It consists of 40% sulphuric acid and 60% distilled water.
The specific gravity of the electrolyte poured into a new batteries with dry charges plate ranges from 1.25 to 1.28 gm/cm3. The level of electrolyte must be 10 to 15 mm above the top of the plate.
When the electrolyte has been added and the battery is given an initial charge then it is ready for operation.
6) Cell connectors
Connector straps connect the negative and positive terminal post of the adjacent cell just above the cell cover.
Each cell of a lead acid battery produce two volts.
Connectors must be heavy enough to carry high current required for starting without overheating.
7) Taper terminals
Battery terminals are of special design made tapered to specified dimension. So that all positive and negative cable clamp will be fit.
The positive terminal are slightly larger in diameter at top than negative terminal.
8) Sealing compound
They are blends of specially processed bituminous substance having resistance to flow at high temperature and resistance to crack at low temperature.
Working of battery
The working of battery can be understood by knowing the charges taking place dining the charging or discharging of the battery.
Chemical reaction during charging and discharging
The chemical reaction takes place between the three chemicals in the battery. In presence of Sulphuric Acid ( H2SO4 ), the electron from one group of plate collect on the other group of plate.
This flow of electrons is continue until there is insufficient in balance of electron to create a two volts pressure between two group of plate.
This results in a pressure of 2 volts between the terminals of the batteries cell. If two terminals are connected by a circuit the electron will flow.
After a certain amount of current has been withdrawn, the battery is discharged or dead. When it is discharged, it is not capable of delivering any aadditional current.It is then charged.
The chemical reaction takes place while the battery is charged and discharged –
PbO2 + 2 H2SO4 + Pb = PbSO4 + 2 H20 + PbSO4 + Q
Lead Peroxide (+) Sulphuric Acid (-) Lead Lead Sulphate (+) Water Lead Sulphate (-) Energy
(Positive plate) (Electrolyte)
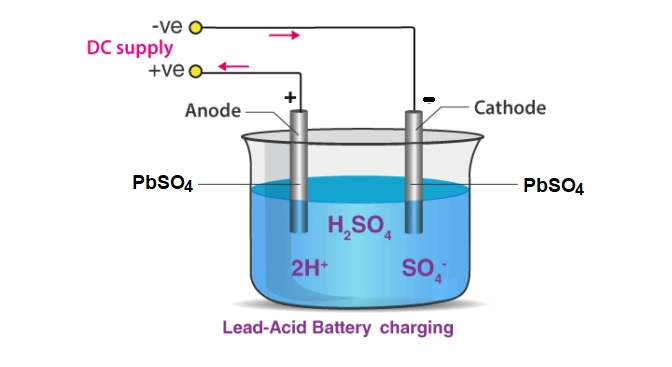
During discharging of battery the sulphuric acid (H2SO4) is split into hydrogen (H2) and sulphate (SO4). The hydrogen liberate at the lead oxide (PbO) which combine with part of sulphuric acid to form lead sulphate (PbSO4) and water.
The sulphate is librated at the spongy lead plates (Pb) and combines with them to form lead sulphate (PbSO4). During this process the electrolyte becomes dilute because of the absorption of SO4 by the sponge lead plates.
When battery is charged, the chemical reaction shown above become reverse. The lead sulphate on the plate again convert to lead peroxide and the lead sulphate on other plate is reduced to spongy lead (Pb). Thus the electrolyte become concentrated because of increase in H2SO4.
Thus the battery convert electrical energy into chemical during charging and chemical energy into electrical energy during discharging.
At higher temperature the chemical reaction operates very vigorously while it is very slow at slow at lower temperature.
Rating of battery
The battery rating are recommended by Society of Automotive engineers
1) 20-hours rating ( in ampere hours)
It indicates the lighting ability of a full charges battery. It is obtained the discharging the battery at a current rate equal to 1/20 of the manufacturer’s ampere hour rating.
The current rate that battery deliver continuously for 20 hours after which cell voltage should not drop below 1.75 and battery temperature is 80 degree F.
2) Cold rating
It gives an indication of the cold weather starting ability of the battery. The number of minutes of a 6 volt battery can deliver 300 ampere at 0 degree F before cell voltage drop below 1 volt.
3) 25-Ampere rating
It measures the battery performance at a moderate constant current output at 80 degree F to a final limiting voltage 1.75 volt/cell.
4) 20 minute rate
It is the amount of current a battery can delivered continuously dining 20 minute without dropping the cell voltage below 1.5. A temperature of 27 degree C is maintained at the start of the test.
Capacity of battery
The capacity of battery has been defined as the amount of current it can deliver.
The maximum amount of current that a cell can furnish is dependent upon the following factor
a) Number of plates
b) Area of plate
c) Temperature of electrolyte
d) Quantity of electrolyte
About 1/10 m2 of the surface plate must be in contact with an electrolyte to produce 40 to 60 ampere of current. 6 volt passenger car battery have 15-17-19 and 21 plates per cell , the 12 volt have 7- 9- 11 and 13 plates per cell depending upon the size of the battery.
There is always one more negative plate then positive plate.
Environmental impact on battery
The environmental impact of batteries is a complex and multifaceted issue that involves various stages of the battery lifecycle, from raw material extraction to manufacturing, use, and disposal.
Different types of batteries have different environmental considerations, and advancements in battery technology continue to impact their overall sustainability.
Raw Material Extraction:
Lithium-ion Batteries: The mining of lithium, cobalt, nickel, and other materials used in lithium-ion batteries can have environmental and social impacts, including habitat destruction, water pollution, and human rights concerns in mining regions.
Lead-Acid Batteries: Lead is a toxic material, and the mining and processing of lead for lead-acid batteries can lead to environmental contamination if not properly managed.
Manufacturing:
The manufacturing process for batteries consumes energy and resources, contributing to greenhouse gas emissions and environmental degradation.
The environmental impact varies depending on the type of battery and the manufacturing practices of the specific manufacturer.
Use Phase:
The use phase of a battery involves the energy consumption and emissions associated with charging and discharging. For electric vehicle (EV) batteries, the use phase is generally more environmentally friendly compared to traditional internal combustion engines, especially if the electricity comes from renewable sources.
End-of-Life:
Proper disposal and recycling of batteries are crucial to minimize environmental impact. Inadequate disposal methods can lead to the release of toxic substances, such as heavy metals, into the environment.
Recycling technologies for certain types of batteries, like lithium-ion batteries, are still developing, and improving recycling rates is essential for reducing environmental impact.
Battery Chemistry:
Different battery chemistries have different environmental profiles. For example, lithium-ion batteries are widely used but have challenges in terms of resource extraction and recycling.
Other types, such as sodium-ion or solid-state batteries, may offer environmental advantages but are still in the early stages of commercialization.
Advancements in Technology:
Ongoing research and development are focused on creating more sustainable and environmentally friendly battery technologies. This includes efforts to reduce reliance on scarce or environmentally damaging materials, improve energy density, and enhance recycling processes.
Policy and Regulation:
Government policies and regulations play a crucial role in shaping the environmental impact of batteries. Regulations regarding recycling, responsible sourcing of materials, and emissions standards can drive improvements in the industry.
Use and maintenance of battery
Proper use and maintenance of batteries are essential to ensure their optimal performance, longevity, and safety. The guidelines for battery use and maintenance can vary depending on the type of battery (e.g., lead-acid, lithium-ion) and the specific application (e.g., automotive, electronic devices).
Here are some general tips:.
Charging:
Follow Manufacturer Guidelines: Adhere to the manufacturer’s recommendations regarding charging voltage, current, and temperature.
Use Compatible Chargers: Use chargers designed for the specific type of battery to prevent overcharging or overheating.
Discharging:
Avoid Deep Discharges: For rechargeable batteries, avoid deep discharges, as frequent deep cycling can reduce battery life.
Temperature:
Operate in Recommended Temperature Range: Batteries have optimal temperature ranges for operation. Avoid exposing batteries to extreme temperatures, both high and low.
Storage:
Store in a Cool, Dry Place: If storing batteries for an extended period, keep them in a cool, dry place. Check and maintain a suitable storage charge level, especially for rechargeable batteries.
Avoid Overheating:
Prevent Overheating during Use: High temperatures can degrade battery performance. Avoid using devices in direct sunlight or hot environments.
Avoid Overcharging:
Disconnect After Charging: Once a battery is fully charged, disconnect it from the charger to prevent overcharging.
Regular Inspections:
Check for Physical Damage: Regularly inspect batteries for signs of physical damage, leakage, or bulging. If any issues are found, replace the battery promptly.
Proper Disposal:
Follow Recycling Guidelines: Dispose of batteries properly, following local regulations. Many batteries contain hazardous materials and should not be disposed of in regular trash.
Use the Right Battery Type:
Match Batteries to Device Requirements: Ensure that the batteries used in a device are compatible and meet the device’s specifications.
Maintenance Charging (Lead-Acid Batteries):
Perform Periodic Maintenance Charging: For lead-acid batteries, especially in vehicles that may be parked for extended periods, perform periodic maintenance charging to prevent sulfation.
Avoid Overloading:
Don’t Exceed Rated Capacity: Do not overload batteries by trying to draw more current than they are designed to handle.
Follow Vehicle Maintenance Guidelines:
For Automotive Batteries: Follow the vehicle manufacturer’s guidelines for battery maintenance and replacement.
Educate Users:
Inform Users of Best Practices: In cases where batteries are used in consumer products, provide users with information on proper battery use and maintenance.
Update Firmware (Smart Batteries):
For Smart Batteries: If using batteries with embedded electronics or firmware (e.g., lithium-ion batteries in laptops), ensure that firmware is up-to-date for optimal performance and safety features.
By following these guidelines, users can maximize the lifespan and efficiency of batteries while minimizing safety risks and environmental impact. Always refer to the specific recommendations provided by the battery manufacturer for the best results.
Batteries in modern cars
Modern cars commonly use lead-acid or lithium-ion batteries, and the choice between the two depends on various factors such as the vehicle’s design, power requirements, and cost considerations.
Here’s an overview of the role and characteristics of batteries in modern cars:
Lead-Acid Batteries:
Type: Traditional lead-acid batteries are still widely used in internal combustion engine vehicles.
Functionality: Lead-acid batteries serve primarily to start the engine (starter battery) and provide power to accessories when the engine is off.
Voltage: Typically, automotive lead-acid batteries are 12 volts.
Maintenance: Some lead-acid batteries may require periodic maintenance, such as checking fluid levels and topping up with distilled water.
Durability: Lead-acid batteries are robust and can handle repeated charging and discharging cycles.
Cold-Cranking Amps (CCA): CCA is a crucial specification for lead-acid batteries, indicating their ability to start an engine in cold temperatures.
Lithium-Ion Batteries:
Type: Lithium-ion batteries are increasingly used in modern cars, especially in hybrid and electric vehicles (EVs).
Functionality: Lithium-ion batteries power the electric motor(s) in hybrid and electric vehicles. In some cases, they may also serve to start the engine in conventional vehicles (start-stop systems).
Voltage: Lithium-ion batteries can have different voltages, but common in automotive applications are high-voltage systems (e.g., 48 volts in mild-hybrid systems).
Maintenance: Generally, lithium-ion batteries are maintenance-free, and they don’t require the same level of attention as lead-acid batteries.
Durability: Lithium-ion batteries are known for their high energy density and long lifespan, but they can degrade over time, impacting their capacity.
Weight and Size: Lithium-ion batteries are lighter and more compact compared to lead-acid batteries with similar energy storage capabilities.
Regenerative Braking: Lithium-ion batteries in hybrid and electric vehicles benefit from regenerative braking, where energy is recaptured during braking and used to recharge the battery.
Temperature Sensitivity: Lithium-ion batteries may be sensitive to extreme temperatures, and proper thermal management is crucial for optimal performance.
Start-Stop Systems:
Many modern internal combustion engine vehicles are equipped with start-stop systems, where the engine turns off when the vehicle is stationary and restarts when the driver accelerates. In these systems, a special type of lead-acid battery, known as an enhanced flooded battery (EFB) or an absorbent glass mat (AGM) battery, is often used to handle the frequent starts and stops.
Overall Trends:
The automotive industry is evolving, with an increasing focus on electrification. Hybrid and electric vehicles are becoming more common, driving advancements in battery technology. Lithium-ion batteries, in particular, play a critical role in powering these vehicles, offering higher energy density and efficiency compared to traditional lead-acid batteries.
It’s important to note that the choice of battery technology depends on the specific requirements of the vehicle, and manufacturers may opt for different solutions based on factors such as cost, performance, and overall vehicle design.
Functions of a car battery
The primary function of a car battery is to provide electrical energy to start the engine. However, a car batteries serves several other functions in a vehicle’s electrical system.
Starting the Engine:
The main function of a car battery is to provide the initial electrical power needed to start the engine. When you turn the key in the ignition, the batteries supplies the necessary current to the starter motor.
Powering Electrical Systems:
While the engine is running, the alternator generates electricity and charges the battery. This stored electrical energy is used to power various electrical systems and accessories in the vehicle, such as lights, radio, air conditioning, power windows, and more.
Voltage Stabilization:
The battery helps stabilize the voltage in the vehicle’s electrical system. It acts as a buffer to absorb voltage spikes and fluctuations, providing a consistent and stable power supply to the electrical components.
Backup Power Source:
In case of alternator failure or when the engine is off, the batteries serves as a backup power source for essential electrical systems. This ensures that critical functions like lights and safety systems remain operational.
Voltage Regulation:
The battery helps regulate the voltage in the electrical system. It provides a stable voltage to ensure proper functioning of electronic components and prevent damage caused by voltage fluctuations.
Powering Electronic Control Modules:
Modern vehicles have numerous electronic control modules that manage various functions, such as engine control, transmission control, and safety systems. The battery supplies power to these modules to ensure proper operation.
Supporting Start-Stop Systems:
In vehicles equipped with start-stop systems, the battery plays a crucial role. It provides power to restart the engine quickly when the vehicle comes to a stop and then starts moving again.
Emergency Power Source:
In emergency situations, a car battery can be used as a power source for charging electronic devices, such as smartphones or other portable devices, using the appropriate adapters.
Maintaining Electrical Memory:
The battery helps maintain the electrical memory of certain vehicle systems, such as the radio presets, clock, and engine control module. This prevents the loss of settings when the engine is turned off.
Storing Energy from Regenerative Braking (Hybrid/Electric Vehicles):
In hybrid and electric vehicles, the battery plays a central role in storing energy generated during regenerative braking. This stored energy can then be used to assist the vehicle during acceleration.
FAQ
Which battery is used in automobile?
Lead Acid Battery
The most common type of car battery in India is a Lead Acid Battery. It comprises many plates placed in an electrolyte solution. This solution is made of 65% water and 35% sulphuric acid. The current is produced by the chemical reaction between the plates and the electrolyte solution.
What are the 3 types of batteries?
1) Lead-Acid battery
2) Alkaline battery
3) Zinc-air battery
What is the basic principle of battery?
A battery is a device that stores chemical energy and converts it into electrical energy. Chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an electrical current that can be used to do work.
What are the 3 basic components of a battery?
There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode; and the electrolyte, which separates these terminals. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and the anode.
Which acid is used in battery?
sulfuric acid
Is a car battery AC or DC?
DC
You may also like
External link
